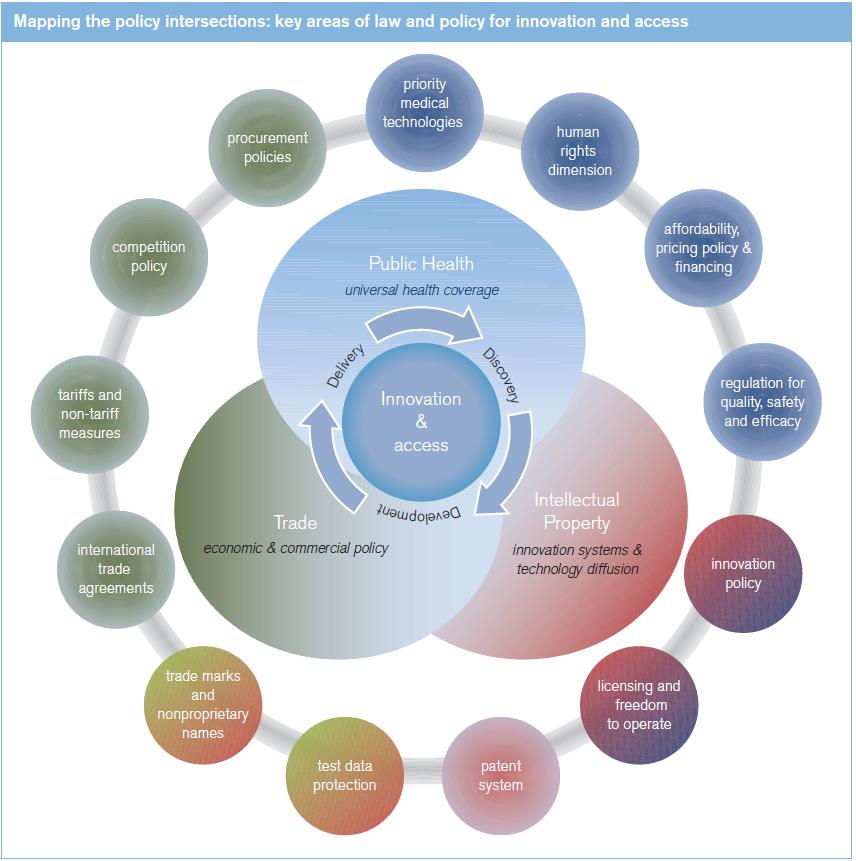
Why this study?
Public health is inherently a global challenge and thus assumes high priority for international cooperation. The World Health Organization (WHO) is the directing and coordinating authority for health, but the interaction between health issues and other policy domains – human rights, development policy, intellectual property (IP) and international trade – creates a strong rationale for cooperation and coordination between the WHO and other international organizations such as the World Intellectual Property Organization (WIPO) and the World Trade Organization (WTO). This study has emerged from an ongoing programme of trilateral cooperation between these agencies. It responds to an increasing demand, particularly in developing countries, for strengthened capacity for informed policy-making in areas of intersection between health, trade and IP, focusing on access to and innovation of medicines and other medical technologies. The need for cooperation and coherence at the international level has intensified over the past decade, as successive multilateral decisions have confirmed (see box).
| Steps towards coherence | |
|
2000 |
United Nations General Comment on the Right to Health |
2001 |
WTO Doha Declaration on the TRIPS Agreement and Public Health |
2002 |
WHO–WTO joint study WTO Agreements and Public Health |
2003/5 |
WTO creates new TRIPS flexibility for access to medicines in countries lacking manufacturing capacity |
2006 |
WHO Commission report on Public health, innovation and intellectual property rights |
2007 |
WIPO Development Agenda |
2008 |
WHO Global Strategy and Plan of Action on Public Health, Innovation and Intellectual Property |
2009 |
WHO–WIPO–WTO trilateral cooperation commences |
The study is set in an evolving health policy context: notably, from an initial focus, a decade ago, on access to medicines for infectious epidemics, debate has broadened to consider innovation policy and a wider range of diseases and medical technologies. Policy-makers increasingly need to understand the complex interplay between different disciplines, at a time when stronger analytical tools and improved data open up new possibilities for this work. An integrated approach can reinforce a dynamic, positive interplay between the measures that promote innovation and those that ensure access to vital medical technologies. While addressing the broader issue of innovation and access to the whole range of medical technologies, the study focuses mainly on the area of medicines for which most practical experience and data are available.
Navigating the study
The study has been prepared as a capacity-building resource for policy-makers. The study is structured so as to enable users to grasp the policy essentials, and then to look more deeply into areas of particular interest. It therefore lays out a general panorama of the policy landscape (see Chapter II), so that all interrelated elements can be seen in context. It then provides more detailed accounts of issues specifically connected with innovation (see Chapter III) and access (see Chapter IV). The contents mirror the evolution of multilateral policy debate over the past decade, recognizing that innovation and access are inevitably intertwined – access without innovation would mean a declining capacity to meet an evolving global disease burden; and innovators need to consider how new technologies can reach those most in need.
- Chapter I presents the general background to health policy relating to medical technologies, sets out the distinct roles and mandates of the three cooperating agencies, and outlines the global disease burden that defines the essential challenge for health policy. In view of the significant contribution to health policy of a range of diverse actors, Annex I describes a selection of entities active in current policy processes.
- Chapter II outlines the essential elements of the international framework – health policy, IP and trade policy – laying an integrated basis for the following more detailed analysis of the innovation and access dimensions. It outlines the key insights of economics for medical technology innovation and access, in view of the growing use of economic concepts to inform health policy discussions. A final section reviews the policy issues associated with traditional medical knowledge, in view of its significance for national health systems and as an input to medical research.
- Chapter III provides a more detailed overview of policy issues concerning the innovation dimension of medical technologies. The historical pattern of medical research and development (R&D) provides a backdrop for analysing current trends in the R&D landscape. The chapter looks at the innovation challenge presented by neglected diseases and related alternative and complementary instruments to promote research and development. It outlines the role of IP rights in the innovation cycle. A final section looks at influenza vaccines as a distinct example of innovation management and product development to address a specific global health need.
- Chapter IV deals with key aspects of the access dimension, describing the context for access to medical technologies and the current access framework for essential medicines. It then sets out the key determinants of access related to health systems, IP and trade. It reviews in particular pricing policies, taxes and mark-ups, and procurement mechanisms, as well as regulatory aspects and initiatives to transfer technology and boost local production, patent quality and review procedures, compulsory and voluntary licences, trade agreements, tariffs and competition policy.
As access and innovation issues are increasingly considered across a broader perspective, a more diverse set of stakeholders, values, experience, expertise and empirical data now shapes and informs policy debates, through: |
|
|
|
The cross-cutting character of these policy domains means that some themes are introduced in Chapter II, in the course of sketching out the general policy framework, and are later elaborated in either Chapter III and/or Chapter IV which look in more detail at how these elements have bearing on innovation and access respectively. For example, the general elements and principles of IP policy are set out in Chapter II, while Chapter III elaborates aspects of IP policy, law and practice that bear particularly on innovation of medical technologies, and Chapter IV considers how specific aspects of IP impact on access to technologies. Similarly, the broad rationale for regulation of medical technologies is set out in Chapter II, and Chapters III and IV deal with the implications of product regulation respectively for the innovation process and for access to medical technologies. Regarding trade policy, Chapter II sets out the main elements and Chapter IV considers the impact of trade and trade policy settings on access to medicines and other medical technologies.
The global disease burden is a moving target, requiring dynamic responses …
Currently, most people in high-income countries live beyond the age of 70 and die of chronic diseases; these are also leading causes of death in middle-income countries, along with tuberculosis, HIV/AIDS and road traffic accidents; but in low-income countries, people predominantly die of infectious diseases and more than a third of all deaths are among persons aged under 15. Large declines in mortality from principal communicable, maternal, perinatal and nutritional causes are projected for 2030. But ageing of populations in low- and middle-income countries (LMICs) will result in more deaths due to non-communicable diseases leading to a double burden of disease. While preventive measures with respect to lifestyle, physical inactivity, tobacco use and harmful use of alcohol, nutrition and environmental factors are key, the innovation system has to adjust to these changes in the global disease burden. The focus on access to medicines – which in the past has been on communicable diseases such as HIV/AIDS and malaria – has broadened. Access to treatments for non-communicable disease, including expensive cancer treatments in middle-income countries, will be the challenge of the future and the focus of the access debate (see Chapter I, Section C).
Access to medicines and the right to health
Access to essential medicines and health services is an element of the fulfillment of the right of everyone to the enjoyment of the highest attainable standard of health. Furthering access to medicines is also part of the Millennium Development Goals (see Chapter II, Section A.1-3). The WHO framework for access to medicines recognizes that lack of access to medical technologies is rarely due to a single isolated factor and thus includes rational selection and use of medicines; affordable prices; sustainable financing; and reliable health and supply systems with quality as an underpinning element (see Chapter IV, Section A.1). Rational selection of the needed medication requires a country to identify which medicines are most important to address the national burden of disease. This selection can be guided by the WHO Model Lists of Essential Medicines. Political commitment to adequate and sustainable funding is a basic condition for effective and sustainable access (see Chapter IV, Section A.1). Affordable prices are a critical determinant of access to medicines, especially in countries where public health sector is weak and where those with most limited means are often required to secure medicines at market prices. Generic competition is a key factor in driving prices down; yet even low priced generic medicines are often still unaffordable for large parts of the population in many LMICs and availability of essential medicines in the public sector is still insufficient (see Chapter IV, Section A). The overarching condition for providing access to needed medical technologies and health services is a functioning national healthcare system (See Chapter II, Section A.5, and Chapter IV, Section B).
Access for HIV/AIDS treatments has been a major focus for policy-makers in recent years. Low prices for generic antiretroviral treatments have helped governments and donor programmes progress towards the goal of having 15 million people on treatment by 2015 (see Chapter IV, Section A.2). Other critical areas are access to and innovation of paediatric formulations and medical devices (see Chapter IV, Sections A.2 and A.3). The changing burden of disease also leads to a greater focus on access and IP issues in relation to non-communicable diseases (see Chapter IV, Section A.2). National immunization programmes are a highly effective public health tool for the prevention of illness and the spread of infectious diseases. Distinct market conditions and know-how requirements create a different landscape for the development and dissemination of vaccines (see Chapter III, Section B.4, and Chapter IV, Section A.2, see also Chapter III, Section E).
Governments explore further measures to contain costs and increase access
Governments employ many different means to cut prices for medical technologies, including direct price controls, reference pricing, and reimbursement limits; and they increasingly use health technology assessments to control costs (see Chapter IV, Section B.1). On top of import tariffs (see Chapter IV, Section D), various taxes (see Chapter IV, Section B.3) and mark-ups along the supply chain (see Chapter IV, Section B.4) also boost consumer prices and constrain access. Removing tariffs and taxes and regulating supply chain distribution mark-ups can lower prices, where passed on to consumers. Yet, price regulation equally needs to ensure sustainable margins for commercial suppliers.
Differential pricing applied by companies can be a complementary tool to increase access, by linking prices to the differing capacity to pay according to income levels within distinct markets (see Chapter IV, Section B.2). Another strategy for enhanced access to medicines stresses developing local production capacity and leveraging technology transfer, which raises issues of access to medicines, economic and commercial factors, and industrial policy (see Chapter IV, Section B.6).
With regard to access to patented products, countries also make use of the flexibilities available under the WTO Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement).
Regulation of technologies is vital in itself, but can impact innovation and access
Regulation of medical technologies addresses essential health policy objectives: products must be safe, efficacious and of adequate quality. Yet, regulation also shapes the landscape for access and innovation: higher safety standards require the generation of more data and thus increase the cost of innovation. Unjustified regulatory barriers and lengthy marketing authorization processes delay access to needed medical technologies (see Chapter II, Section A.6). Most clinical trials are carried out by or on behalf of the companies developing the tested products. The registration of these trials is a scientific and ethical responsibility and therefore WHO runs the International Clinical Trials Registry Platform. From the perspective of public health policy, clinical trial results should be publicly available, so that researchers and other interested groups themselves can assess the efficacy and potential side effects of new products (see Chapter III, Section B.5). The emergence of biological medicines has raised challenges for established regulatory systems, notably how to regulate "biosimilar" follow-on products (see Chapter II, Section B.6) while still sufficiently incentivising originator companies.
Another challenge for regulatory systems is the steady increase of substandard and spurious/falsely-labelled/ falsified/counterfeit (SFFC) medical products that are posing serious public health problems globally, and especially in regions where the regulatory and enforcement systems are weak. To effectively combat substandard and SFFC medicines a mix of measures is required. Enforcement of good manufacturing practice standards is required to eliminate substandard products while to fight SFFC products additional measures are needed, including border controls and criminal law along with collaboration between legislative bodies, enforcement agencies and courts at the national and international levels (see Chapter II, Section B.1, and Chapter IV, Section B.7).
Overall, regulators face the challenge to balance the benefit of the early release of new products with safety concerns and to define an acceptable level of risk. The need to simplify regulation while maintaining its stringency and cost-effectiveness requires more coordination through regional and international regulatory mechanisms, so as to enable suppliers to service regional markets without undue regulatory complexity or cost (see Chapter II, Section A.6). Full international harmonization of regulatory standards remains an elusive goal. The WHO Prequalification Programme has greatly facilitated the access to quality medical products in developing countries (see Chapter IV, section B.7).
Innovation in medical technologies operates in a complex, fast evolving policy framework …
Innovation in medical technologies requires a complex mix of private and public sector inputs; it differs from innovation in general due to the ethical dimension of medical research, a rigorous regulatory framework, liability questions, and the high cost and high risk of failure. Economic, commercial, technological and regulatory factors have precipitated rapid change in the current landscape for R&D, involving more diverse innovation models and a wider range of active players. Providing specific incentives to absorb the high cost and associated risks and liabilities is a central policy challenge; this has been the historic role of the patent system in particular as applied to pharmaceuticals. While estimates vary of the actual cost of medical research and product development, innovation is undoubtedly costly and time consuming. The risk and uncertainty of innovation increases R&D costs in this sector, as the cost of products that fail to clear regulatory hurdles to become commercialized products has to be added (see Chapter III, Section B.3). Rising expenditure for medical research has not been matched by a proportionate increase in new products entering the market, sparking a debate about research productivity and a quest for new models of innovation and for financing R&D. Many initiatives are exploring new strategies for product development, thus informing a rich debate about how to improve and diversify innovation structures to address unmet health needs. Current policy discussions have reviewed possibilities for open innovation structures, and a range of push and pull incentives, including schemes such as prize funds that would delink the price of products from the cost of R&D (see Chapter III, Section C.2).
… sparking new thinking on industry's role and structure, and on the public/ private divide
This evolving innovation landscape is driving change in the pharmaceutical industry; driving factors include tighter government health budgets; non-profit entities engaged in medical research and product development; the exposure to stricter product regulation and greater liability risks; new technologies enabling targeted treatment; and the greater share of global demand from emerging markets. The historic industry model of vertically integrated in-house R&D and exclusive marketing is opening up to more diverse and collaborative structures, with major industry players developing products by integrating technologies sourced elsewhere, either licensed in or acquired through mergers and integration of smaller firms. Research-based firms have also invested in generic production capacity. The role of public research and academic institutions, increasingly in developing countries, has also come under the spotlight as they seek to reconcile public interest responsibilities with the need for private sector partnerships to deliver new medical products (See Chapter III, Sections A and B, and Chapter II, Section C).
Neglected diseases: a policy challenge but a growing focus of practical initiatives
Market-based innovation models fail to address the disease burden specific to developing countries, the so-called neglected diseases. Since this research gap has been identified, the landscape of health research for these diseases has evolved. Product development partnerships (PDPs) have been a significant development over the past decade, drawing together not-for-profit entities and industry players, with major philanthropic funding, significantly increasing the number of products in development for neglected diseases, and identifying pathways regarding existing research gaps (see Chapter III, Section C.4). Pharmaceutical research based companies also engage increasingly in philanthropic research. Several companies have established dedicated research institutes to research on diseases disproportionately affecting developing countries or participated in cooperative projects to share assets and knowledge, such as WIPO Re:Search, which has been developed to make better use of IP protected assets and improve access (See Chapter III, Sections C.5-6). However, much more needs to be done by the international community in this area. The WHO Consultative Expert Working Group has recommended that negotiations begin on a globally binding treaty on R&D for neglected diseases. The recommendations of the Group were discussed by WHO member states in an intergovernmental meeting in November 2012 (see Chapter III, Section C.3).
The IP system at the centre of debate on innovation and access …
Several elements of the IP system touch both on innovation and on access (see Chapter II, Section B.1).
The focus has been on the patent system and test data protection, but other relevant aspects of IP include the relationship between trademarks and international nonproprietary names (INN) and copyright questions regarding the package insert of medicines (see Chapter II, Section B.1). The patent system has been widely used for medical technologies especially by the pharmaceutical sector. Indeed, the pharmaceutical sector stands out in terms of its dependence on patents to capture returns to R&D, but its role in innovation and how to enhance its effectiveness are matters of continuing debate (see Chapter III, Section B). Patents, in principle, promote innovation by providing incentive to invest in R&D, a particular consideration for the private sector. Patents function to structure, define and build innovation partnerships. The impact of patents on access is complex and an area of particular focus: policy options mean that the mere existence of a patent need not be an absolute barrier to access, but equally the absence of an enforceable patent right does not guarantee effective access (see Chapter IV, Section C).
The TRIPS Agreement sets minimum standards for IP protection and enforcement. For example, patents must be available for any innovations in all fields of technology, provided they are new, involve an inventive step (or are non-obvious) and are capable of industrial application (or are useful). The role of intellectual property rights in the innovation cycle is addressed in Chapter III, Section D. Strict patentability criteria and strict patent examination supported by patenting examination guidelines contribute to prevent strategies employed to delay the entry of generic competition, such as "evergreening" (see Chapter III, Section D.3, and Chapter IV, Section C.1). Integral to the patent system is the requirement to make accessible such innovation through public disclosure, thus creating an extensive knowledge base. The resultant patent information serves as a tool for charting freedom to operate, potential technology partnerships, and procurement options, as well as giving policy-makers insights into patterns of innovation (see Chapter IV, Section B.5). Patent information is more accessible in general, but coverage of data concerning many developing countries remains a challenge. Recent trends show a growth in patent applications on medical technologies from a more diverse range of public and private entities, and from key emerging economies (see Chapter II, Section B.1).
The protection of clinical trial data also illustrates the complex relationship between the IP system and innovation and access. Protecting these data against unfair commercial use is important given the considerable efforts made to generate these data and thus bring new medicines to the market. On the other hand, certain forms of test data protection potentially delay the entry of generic medicines. The TRIPS Agreement requires protection of test data, but does not specify the exact form it should take, and national authorities have taken diverse approaches (see Chapter II, Section B.1).
How patents are licensed can determine their impact on public health …
Appropriate licensing of patents can help build partnerships and enable innovation through cooperation to bring new medical technologies to fruition. Private sector licensing strategies typically aim at commercial objectives, but public sector entities can use patents expressly to leverage public health outcomes. New models of socially responsible licensing protect IP while ensuring that new medical technologies are available and affordable for underserved communities. Public– private partnerships have resulted in creative licensing agreements that forgo profit maximization in favour of providing essential technologies to poorer countries at affordable prices. Voluntary licences also form part of corporate social responsibility programmes, especially for HIV/AIDS treatments. The Medicines Patent Pool has reinforced the trend towards voluntary licensing programmes that increase access to medicines by enabling new formulations and enhancing provision of cheaper generic medicines for developing countries (see Chapter IV, Section C.2).
… as do policy options and IP flexibilities
A wide range of policy options and flexibilities are built into the international IP regime that can be used to pursue public health objectives. These options are not self-actuating at the international level, though, and attention and action are needed at the domestic level as to how best to implement such flexibilities, so that the national IP regime responds to each country's individual needs and policy objectives. Key options include transition periods for LDCs (see Chapter II, Section B.1), differing IP exhaustion regimes, refining the criteria for grant of a patent, pre-grant and post-grant opposition procedures, as well as exceptions and limitations to patent rights once granted, including regulatory review exception ("Bolar" exception) to facilitate market entry of generics, compulsory licences and government use. Countries have used these instruments to improve access to medicines for both communicable and noncommunicable diseases (see Chapter IV, Sections C.1-3). WTO members have agreed to amend the TRIPS Agreement to permit a wider use of compulsory licensing for access to medicines, clearing a potential legal barrier for countries that need to import medicines produced abroad under a compulsory licence, through the grant of special compulsory licences for export under what is termed the "Paragraph 6 System" (see Chapter IV, Section C.2, and Annex II). While the legal scope for flexibilities is now clearer, thanks also to the Doha Declaration on Public Health, and some flexibilities are widely implemented (such as "Bolar" exceptions), policy debate continues on the use of measures such as compulsory licensing.
International trade is an essential avenue to access, but does not eliminate economic disparities
International trade is vital for access to medicines and other medical technologies, markedly so for smaller and less resourced countries. Trade stimulates competition, which in turn reduces prices and offers a wider range of suppliers, improving security and predictability of supply. Trade policy settings, such as tariffs on medicines, pharmaceutical ingredients and medical technologies, therefore directly affect their accessibility (see Chapter II, Sections B.3-5, and Chapter IV, Section D). Trade policy and the economics of global production systems, are also key factors in strategic plans to build domestic production capacity in medical products. Nondiscriminatory domestic regulations founded on sound health policy principles are also important for a stable supply of quality health products. Access to foreign trade opportunities can create economies of scale to support the costs and uncertainties of medical research and product development processes.
Developed countries have dominated trade in health-related products but India and China have emerged as leading global exporters of pharmaceutical and chemical inputs, and some other developing countries have shown strong recent export growth. Countries' imports of health related products differ dramatically according to level of development, illustrating substantial and widening gaps in access: over recent years, LDC imports have grown least, starting from a low base.
Import tariffs on health-related products can affect access: since they increase cost early in the value chain, their impact on price may be magnified. Developed countries have largely eliminated such tariffs, in line with a WTO deal on pharmaceutical trade. Other countries have reduced tariffs significantly, but the picture is still mixed: some developing countries structure tariffs to promote local production, while LDCs apply lower tariffs (see Chapter IV, Section D.1).
Competition policy promotes effective innovation and helps shape the conditions for access
Competition policy is relevant to all stages in the process of supplying medical technology to patients, from their development to their sale and delivery. The creation of sound competitive market structures through competition law and enforcement has thus an important role to play in enhancing both access to medical technology and fostering innovation in the pharmaceutical sector. It can serve as a corrective tool if IP rights hinder competition and thus constitute a potential barrier to innovation and access. Competition authorities in several jurisdictions have taken action to address anticompetitive practices in the pharmaceutical sector, including some patent settlements, certain licensing practices and pricing policies. Competition policy also has an important role to play in preventing collusion among suppliers of medical technology participating in procurement processes (see Chapter II, Section C.2, and Chapter IV, Section D.2).
Access to medical technologies through more effective government procurement
Access to medical technologies in many countries largely results from government procurement, with pharmaceuticals made available through public funds or subsidies. Procurement systems aim to obtain medicines and other medical products of good quality, at the right time, in the required quantities, and at favourable costs. These principles are particularly important in the health sector given the large expenditures, health impact of value for money and quality issues, with some programmes reportedly paying considerably more than necessary for medicines (see Chapter IV, Section B.5). Procurement policies favouring open and competitive tendering become increasingly important in a fiscal climate when national budgets are under pressure, and philanthropic programmes confront funding constraints. Good governance in procurement is consistent with increasing access to medical technologies through lower prices and uninterrupted supply. The WTO's plurilateral Government Procurement Agreement provides an international framework of rules to promote efficiency and good governance in public procurement, with particular application to procurement of medicines, promoting transparency, fair competition and improved value for public expenditure (see Chapter II, Section B.4).
Free trade agreements beyond the multilateral sphere have increasing relevance for access issues
The international policy and legal framework has been made more complex by the recent growth of trade and IP agreements, outside the established multilateral forums. Policy debate has focused on intellectual property and pharmaceutical regulation measures in these agreements, and their impact on access to medicines. For example, patent term extensions, data exclusivity and other measures such as patent linkage contained in certain free trade agreements are designed to incentivize innovation, but also have the potential to affect access to medicines by delaying the market entry of generic products (see Chapter IV, Section C.5). These agreements also set standards in other policy areas with implications for access, notably standards established on government procurement and competition policy, as well as preferential tariffs on pharmaceuticals, inputs, and other health products (see Chapter II, Section B.5, and Chapter IV, Section C.5). The overall impact of this trend for the international system is yet to be systematically analysed, in particular the full implications of the entire range of such agreements for access to medical technologies.