COVID-19 AND WORLD TRADE
More
An infographic complemented by a checklist of questions identify various trade-related issues involved in the development and distribution of vaccines against COVID-19, from regulatory approval to manufacturing and border clearance and distribution. These non-exhaustive graphics complement the WTO's COVID-related trade monitoring work, which includes the series of information notes on different trade issues related to the pandemic.

As countries are currently working on obtaining regulatory approval for vaccines against COVID-19, the manufacturing and delivering phases will be challenging in scale, reach and complexity. By stressing the interplay between a vaccine's life cycle and trade-related policies, the graphics aim to assist authorities in identifying trade-related best practices, including regulatory.
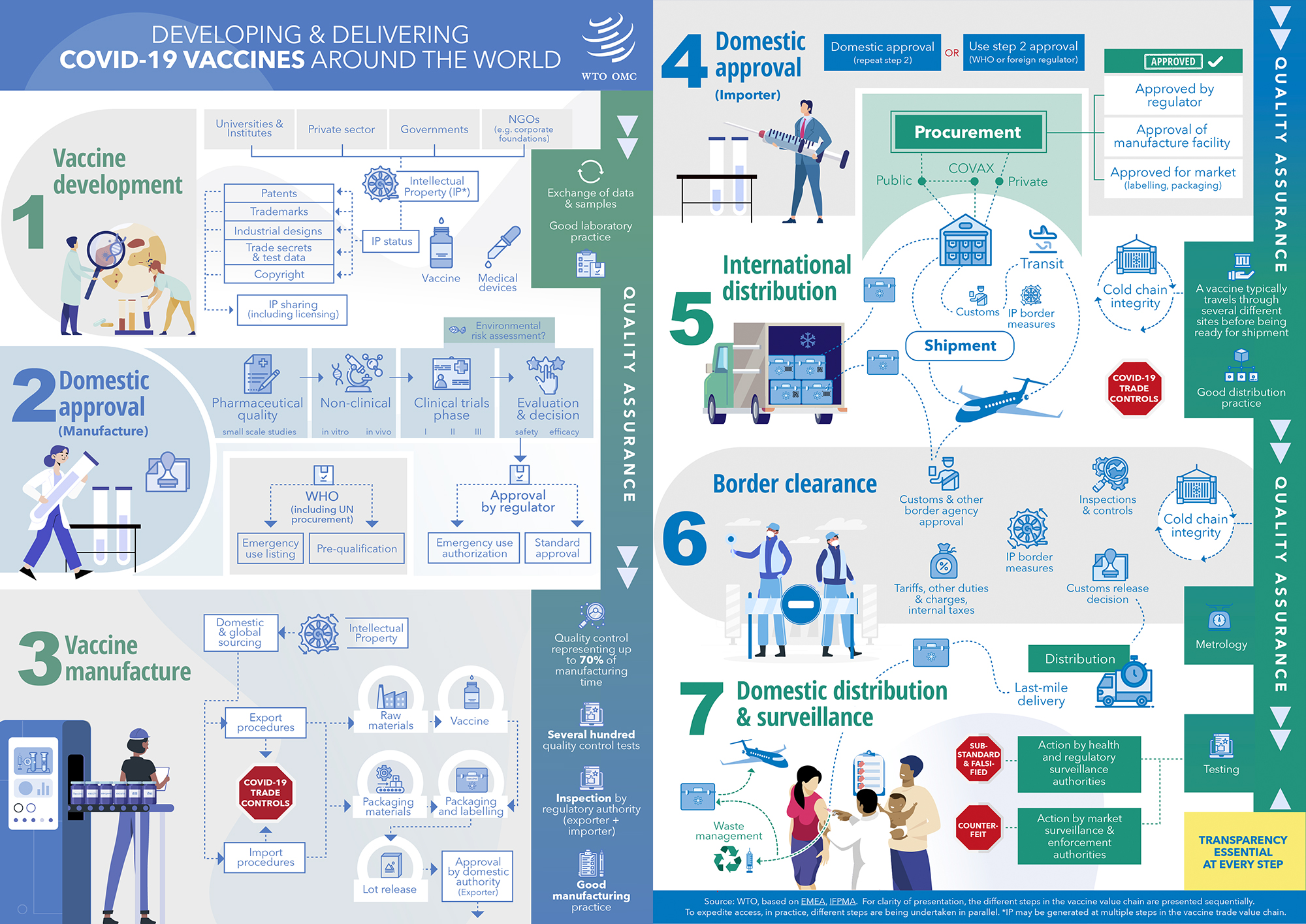
They invite reflection on which laws and regulations could help facilitate access to the vaccines across the global value chain, including in transit — for example, measures to reduce costs, facilitate technology transfer, as well as to simplify and expedite authorization, quality assessment and licensing procedures.
Some potential best practices outlined include exchanging scientific information across borders — including on intellectual property issues — and making information related to compliance issues and regulatory approval available. The graphics note that transparency is essential at all stages and emphasize the importance of effective cooperation between customs and other appropriate authorities.
Among the tools available, the graphics point to the WTO's Trade Facilitation Agreement and the temporary exemption of patent rights related to pharmaceutical products for least-developed countries.
- Infographic: Developing & delivering COVID-19 vaccines around the world
- Developing & delivering COVID-19 vaccines around the world: A checklist of issues with trade impact
Share
Share
Problems viewing this page? If so, please contact [email protected] giving details of the operating system and web browser you are using.